Chlorine and chloramines are both effective disinfectants used in water distribution systems. Each has its own advantages and disadvantages, and the debates over their tradeoffs have gone on for decades.
Most customers can’t tell you which one is used in their system.
As long as systems use one of these disinfectants, there should be no serious problems maintaining residual concentrations, if the systems are managed well. With the patchwork of water systems that exist, with some producing their own water in some purchasing water from neighboring systems, it is not uncommon for systems to have both disinfectants in some locations in one system.
Unfortunately, chlorine and chloramine don’t play well together.
To understand why we need to look at their chemistry. Chloramines (also called combined chlorine) are formed when chlorine and ammonia are mixed in the correct proportion. By weight, the ratio should be 3 to 5 mg of Cl per 1 mg of ammonia. This reaction is described as
NH3 + HOCl à NH2Cl + H2O
(monochloramine)
Monochloramine is a good chloramine and an effective disinfectant. (HOCl is hypochlorous acid which is essentially dissolved chlorine.)
However, when chlorinated and chloraminated water meet in water distribution systems, it is usually impossible to control their stoichiometry.
It is easy to end up with something that looks like this
NH3 + 3 HOCl à NCl3 + 3H2O
(trichloramine or nitrogen trichloride)
Unfortunately, nitrogen trichloride has very little value as a disinfectant. At the meeting of the two disinfectants, there is very little disinfecting occurring. The answer to the question of “Which one wins?” is “nobody”.
This zone of low disinfectant is dynamic and moves back and forth through the day as tank levels go up and down, demand increases and decrease, and pumps turn on and off.
As an operator, what you would like to know in your system is which customers are getting chlorine and which are getting chloramine at any time. Your hydraulic model can help with this. Depending on how detailed you want to be, you can use the source trace option or constituent analysis options in OpenFlows WaterGEMS (and OpenFlows WaterCAD).
For those that really want to get into the chemistry, it’s also possible to use the WaterGEMS multi-species (MSX) analysis feature.
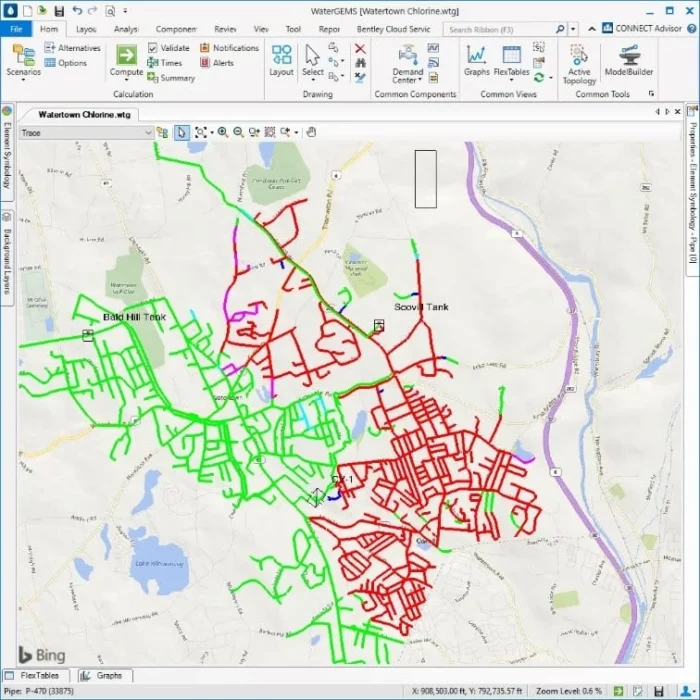
WaterGEMS can provide the operators with a view of which source contributes water to each part of the system at any time throughout the day. They may want to test the operation of valves to control which customers receive which water. They can verify their model by sampling along the border between the two water sources using free chlorine and combined chlorine measurements. If the model is well- calibrated, it can almost tell the street address of the boundary. The red pipes below show water from the chloramine source, while the green pipes show water from the chlorine source.
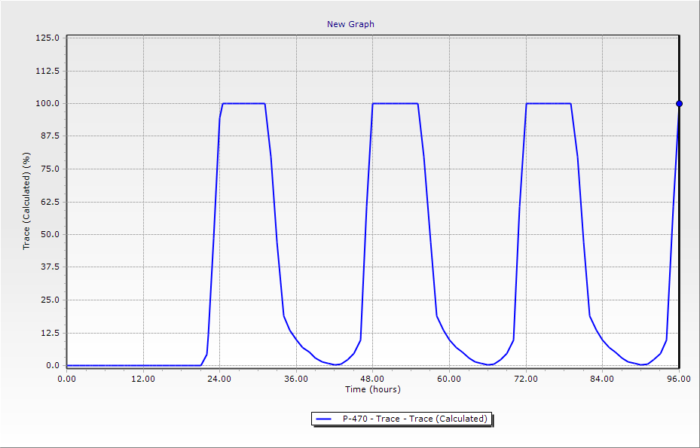
WaterGEMS can calculate how much of the water from each water source reaches each pipe at every time. For most customers, this is not exciting as they get water from the same source all day. However, for others, they can receive 100% of their water for part of a day from one source, and 100% of their water from another source for part of the day with fairly rapid transitions as shown below. The MSX feature can calculate the concentrations of each species but requires a good bit more setup than simple source training, which is usually sufficient.
If you like blogs like this, you can go back to our library of blogs at https://blog.bentley.com/category/hydraulics-and-hydrology/.
Want to learn more from our resident water and wastewater expert? Join the Dr. Tom Walski Newsletter today!